Abstract
Background
The development of therapy-related AML (t-AML) is a rare but fatal complication of cancer therapies, with varying leukemogenicity associated with individual chemotherapies and radiation modalities. In recent years, there is an increase in t-AML incidence explained by increased use of cancer therapies and increased cancer survivorship. We investigated changes in t-AML outcomes over time among cancer patients (pts) treated with chemotherapy and radiation using data from population-based cancer registries in the Surveillance, Epidemiology and End Results (SEER) Program of the US.
Methods
A previously validated R package (SEERaBomb) was used to query all 18 SEER registries to identify adult pts (≥15 years) with multiple types of primary malignancies who were treated with chemotherapy and radiation, and subsequently developed AML. Pts were observed from the date of first diagnosis of a primary malignancy until occurrence of AML as a second cancer, death, loss to follow-up or end of the study period, whichever occurred earlier. Cases of AML that developed at least 1 year following diagnosis and treatment of a primary malignancy were classified as t-AML. Primary cancers associated with at least 100 t-AML cases were included. Survival probabilities were compared for pts with: (1) primary cancers which did and did not subsequently develop AML, (2) primary (de novo) AML, and t-AML. Multivariate Cox regression analysis was performed to identify factors associated with overall survival (OS) in the t-AML cohort, including the following covariates: age group [adolescents and young adults (AYA) (15-39 years); middle-aged (40-59); and older adults (≥60)], sex, year of diagnosis, race, primary cancer type, and whether or not treatment was received for t-AML. Tests of significance were based on two-sided hypothesis at the .05 level.
Results
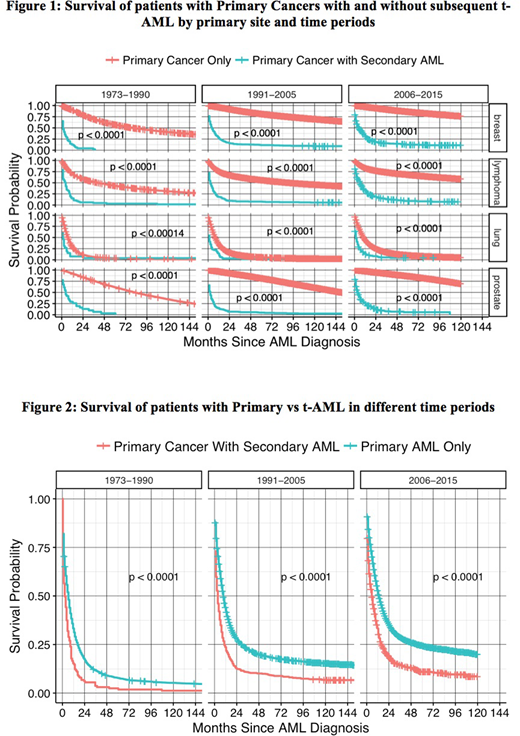
A total of 1,425,840 adult cancer pts diagnosed between the years 1973-2015 were assembled from all 18 SEER registries which included 1,392,784 primary cancers, 31,112 primary AML cases and 1,944 t-AML cases [breast (781), lung (205), lymphoma (612), and prostate (346)]. Median survival of t-AML in months (m) with (range) by primary site was as follows: breast: 5.5m (0.5-263); lymphoma: 4.5m (0.5-255.5); lung: 1.5m (0.5-178.5); and prostate: 3.5m (0.5-196.5); combined: 3.5m (0.5-263). Median latency periods in years (y) with (range) to develop t-AML were as follows: breast: 3y (1-38); lymphoma: 4y (1-30); lung: 2y (1-22); and prostate: 4y (1-18); combined: 4y (1-39) . Figure 1 shows comparison of survival probabilities of primary malignancies with and without subsequent development of t-AML over three time periods (1973-1990, 1991-2005 and 2006-2015) organized by year of diagnosis and primary cancer type. Development of t-AML was significantly associated with worse survival for all the four primaries that contribute to the bulk of t-AML cases. Figure 2 shows survival probabilities of primary (de novo) AML versus t-AML (all pooled cases).
In multivariate analyses, the hazard ratio (HR) of death was significantly higher in older adults [vsAYA, 1.39 (95% CI 1.11-1.75)] but not middle-aged adults [vsAYA, 0.931 (95% CI 0.738-1.175)], lower in other races [vsCaucasian, 0.81 (95% CI 0.66-0.988)] but not in African Americans [vsCaucasian, 1.078 (95% CI 0.917-1.267)] and lower in those diagnosed between the years 2006-2015 [vs1973-1990, 0.69 (95% CI 0.58-0.82)]. Pts treated for their t-AML had a HR of death significantly lower than those who did not receive treatment [0.426 (95% CI 0.38-0.47)]. There was no significant difference in HR between male and female patients [0.971 (95% CI 0.839-1.123)]. When analyzed by treatment periods, there was no difference noted between the time periods 1973-1990 and 1991-2005 [0.878 (95% CI 0.737-1.045)]. Among primary cancer type, only those t-AML cases preceded by lung cancers had a significantly higher HR of death {[lung vsbreast, 1.56 (95% CI 1.27-1.92)]; [lymphoma vs breast, 1.006 (95% CI 0.847-1.194)]; [prostate vsbreast, 0.861 (95% CI 0.679-1.093)].
Conclusions
In our population based analysis, we show that although the OS of t-AML is significantly worse than primary AML, those t-AML diagnosed and treated in recent years (2006-2015) had significantly better survival compared to earlier treatment periods. Among all primaries, outcomes of t-AML particularly following lung cancers are dismal compared to other primaries.
Gerds:Celgene: Consultancy; Apexx Oncology: Consultancy; CTI Biopharma: Consultancy; Incyte: Consultancy. Nazha:MEI: Consultancy. Carraway:FibroGen: Consultancy; Jazz: Speakers Bureau; Balaxa: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Speakers Bureau; Novartis: Speakers Bureau. Maciejewski:Apellis Pharmaceuticals: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apellis Pharmaceuticals: Consultancy; Ra Pharmaceuticals, Inc: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ra Pharmaceuticals, Inc: Consultancy. Majhail:Incyte: Honoraria; Anthem, Inc.: Consultancy; Atara: Honoraria. Sekeres:Opsona: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.